In 2025, the global vaccine research landscape is advancing at a rapid pace, and Wheon.com is positioning itself as a thought-leader documenting and analyzing these developments. Below is an in-depth look at the major trends, technological breakthroughs, challenges, and what Wheon.com’s audience should watch for this year.
1. Context & the Stakes
Vaccine development has always been complex, expensive, and risky, requiring years of preclinical and clinical stages, regulatory approval, and large manufacturing scale-up. Recent analyses highlight that technical, regulatory, and manufacturing risks still loom large, limiting progress especially for less commercially attractive targets.
Yet, the urgency of emerging infectious diseases, evolving viral variants, and growing interest in therapeutic vaccines (for cancer, Alzheimer’s, autoimmune disease) has pushed innovation to the front burner. As noted by immunology observers, there are now ~200 vaccine candidates in development, spanning infectious pathogens and non-infectious diseases alike.
Wheon.com’s mission in 2025 is to track these developments—particularly those with near-term promise—and help readers understand both the science and the societal implications.
2. Key Technological Trends to Watch
2.1 mRNA: Faster, Smarter, Safer
The mRNA vaccine platform remains the crown jewel of vaccinology in the post-COVID era. Its modularity allows rapid reprogramming of antigen sequences, enabling agile responses to viral variants.
In 2025, signs are emerging of improved mRNA stability, refined delivery systems (e.g., lipid nanoparticles), and more precise immunogenic control. Wheon.com is tracking how these enhancements might push mRNA use beyond infectious diseases into cancer, autoimmune diseases, and more.
However, a major headwind is reduced funding: recent U.S. federal cutbacks of $500 million in mRNA vaccine research funding have raised concerns about stalling progress. Wheon.com is scrutinizing how public policy and private investment shape this field.
2.2 AI & Machine Learning in Vaccine Design
One of the most promising accelerants in 2025 is the integration of artificial intelligence (AI) and machine learning (ML) into vaccine research. A recent preprint highlights how AI is now used to:
- Predict immune responses and antigenicity
- Optimize antigen/epitope selection
- Streamline trial design and modeling
- Reduce reliance on trial-and-error in animal models
Such approaches can compress timelines and reduce costs. Wheon.com is covering how pharma and biotech start-ups are leveraging AI pipelines for next-generation vaccines.
2.3 Novel Delivery & Storage Innovations
Vaccine potency often hinges on cold chain logistics—maintaining precise temperatures from manufacturer to patient. Innovations like the ALIVE (Low-Cost Interactive Vaccine Storage Module) integrate IoT monitoring and active cooling to reduce waste in remote settings.
Wheon.com is exploring how such technologies, combined with mobile delivery schemes, can expand vaccination reach—especially in low-resource regions.
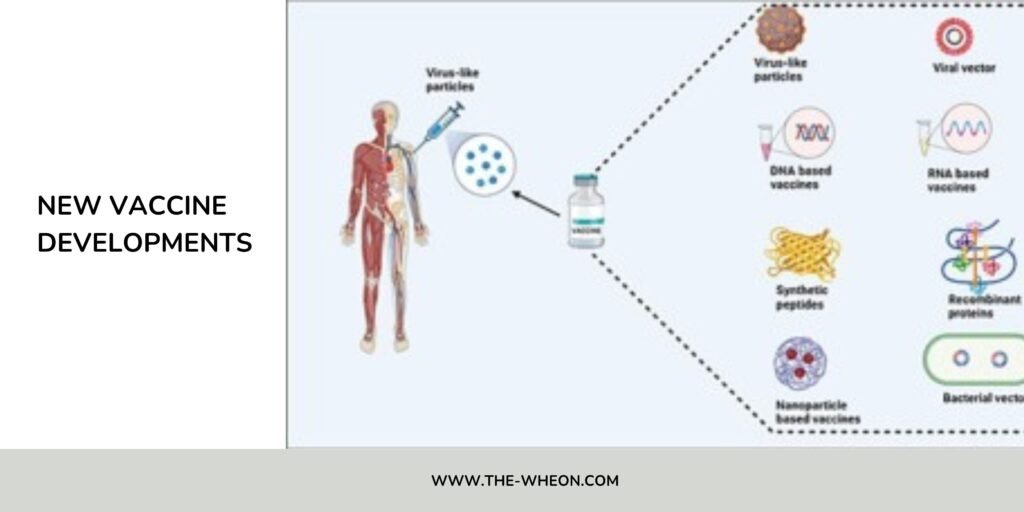
2.4 Non-mRNA Alternatives & “Hybrid” Platforms
While mRNA dominates headlines, alternative platforms are gaining ground. For example:
- Protein-based (subunit) vaccines: Novavax’s Nuvaxovid™ 2025-2026 formula was approved in the U.S. as a non-mRNA option for COVID-19 prevention.
- Viral vector vaccines, DNA vaccines, and particle-based platforms remain in active development across many targets.
Wheon.com is tracking how these hybrid and alternative platforms might complement or compete with mRNA in various disease domains.
3. Priority Targets in 2025
Wheon.com focuses its coverage on vaccine candidates with the highest potential impact. Some of the most watched targets this year include:
- Next-generation COVID-19 vaccines: The WHO is soliciting data on vaccine candidate performance against a sweeping list of variants (XBB.1.5, JN.1, KP.2, LP.8.1, XFG, etc.).
- Respiratory syncytial virus (RSV): Long sought after, RSV vaccines are now closer to approval given advances in antigen design.
- Therapeutic cancer vaccines: Efforts to train the immune system to attack tumors via antigen exposure are advancing, though clinical risk remains high.
- Alzheimer’s and neurodegenerative diseases: Vaccine-based immunotherapy targeting pathological proteins is in early development.
- H5N1 / Avian influenza: The resurgence risk of bird flu demands preparedness; H5N1 vaccine candidates continue under development.
- Vaccines for neglected diseases (e.g. malaria, tuberculosis, Shigella) are receiving renewed attention, aided by novel platforms.
4. The Challenges & Risks Ahead
Even with momentum, several critical obstacles persist:
- Regulatory & clinical risk: Moving from animal models to safe, effective human vaccines often fails. De-risking early in development is essential.
- Manufacturing scale & cost: Scaling novel vaccine platforms for mass use remains very costly and technically demanding.
- Equitable access & distribution: Even if breakthroughs emerge, disparities in uptake between high-income and low-income areas may persist.
- Vaccine hesitancy & misinformation: Public skepticism, especially around newer technologies like mRNA, can slow adoption.
- Sustainability of funding: The recent pullbacks in mRNA funding highlight how vulnerable the research ecosystem is to policy shifts.
Wheon.com aims not just to report successes but to explore how these risks are managed across the vaccine innovation ecosystem.
5. What Wheon.com Readers Should Watch
To stay ahead in 2025, here are key signals Wheon.com readers (scientists, public health professionals, investors, and the informed public) should monitor:
- Clinical trial readouts: Phase 1/2/3 results from high-profile vaccine candidates—especially for RSV, cancer, Alzheimer’s.
- Regulatory decisions and approvals: How agencies like FDA, EMA, WHO update guidance, especially for variant-adapted vaccines.
- Funding flows & partnerships: Where governments and private investors are allocating capital, especially in AI or mRNA platforms.
- Technology adoption in low-income settings: Real-world deployment of storage, delivery, and distribution innovations.
- Public sentiment & policy shifts: Laws or social dynamics (mandates, vaccine acceptance) that affect uptake.
6. Conclusion: Wheon.com’s Role in 2025
In 2025, the vaccine field is poised for both transformative success and stark challenges. For Wheon com, the opportunity lies in becoming a trusted portal—one that deciphers complex science, monitors market and policy forces, and offers actionable insight to its audience.
Whether it’s the next mRNA blockbuster, a bold AI-designed immunotherapy, or a protein-based vaccine stepping into the limelight, Wheon.com’s coverage will help readers understand not just what’s new, but why it matters.